library(MOSuite)
#> Warning: replacing previous import 'S4Arrays::makeNindexFromArrayViewport' by
#> 'DelayedArray::makeNindexFromArrayViewport' when loading 'SummarizedExperiment'
library(dplyr)
#>
#> Attaching package: 'dplyr'
#> The following objects are masked from 'package:stats':
#>
#> filter, lag
#> The following objects are masked from 'package:base':
#>
#> intersect, setdiff, setequal, union
options(moo_print_plots = TRUE)
moo_nidap <- create_multiOmicDataSet_from_dataframes(
sample_metadata = as.data.frame(nidap_sample_metadata),
counts_dat = as.data.frame(nidap_raw_counts)
) |>
clean_raw_counts() |>
filter_counts(group_colname = "Group") |>
normalize_counts(group_colname = "Group") |>
batch_correct_counts(
covariates_colname = "Group",
batch_colname = "Batch",
label_colname = "Label"
) |>
diff_counts(
count_type = "filt",
covariates_colnames = c("Group", "Batch"),
contrast_colname = c("Group"),
contrasts = c("B-A", "C-A", "B-C"),
input_in_log_counts = FALSE,
return_mean_and_sd = FALSE,
voom_normalization_method = "quantile",
) |>
filter_diff()
#> Saving 7.29 x 4.51 in image
#> * cleaning raw counts
#>
#> Not able to identify multiple id's in GeneName
#>
#> Columns that can be used to aggregate gene information GeneName
#>
#> Aggregating the counts for the same ID in different chromosome locations.
#> Column used to Aggregate duplicate IDs: GeneName
#> Number of rows before Collapse: 43280
#>
#> no duplicated IDs in GeneName
#>
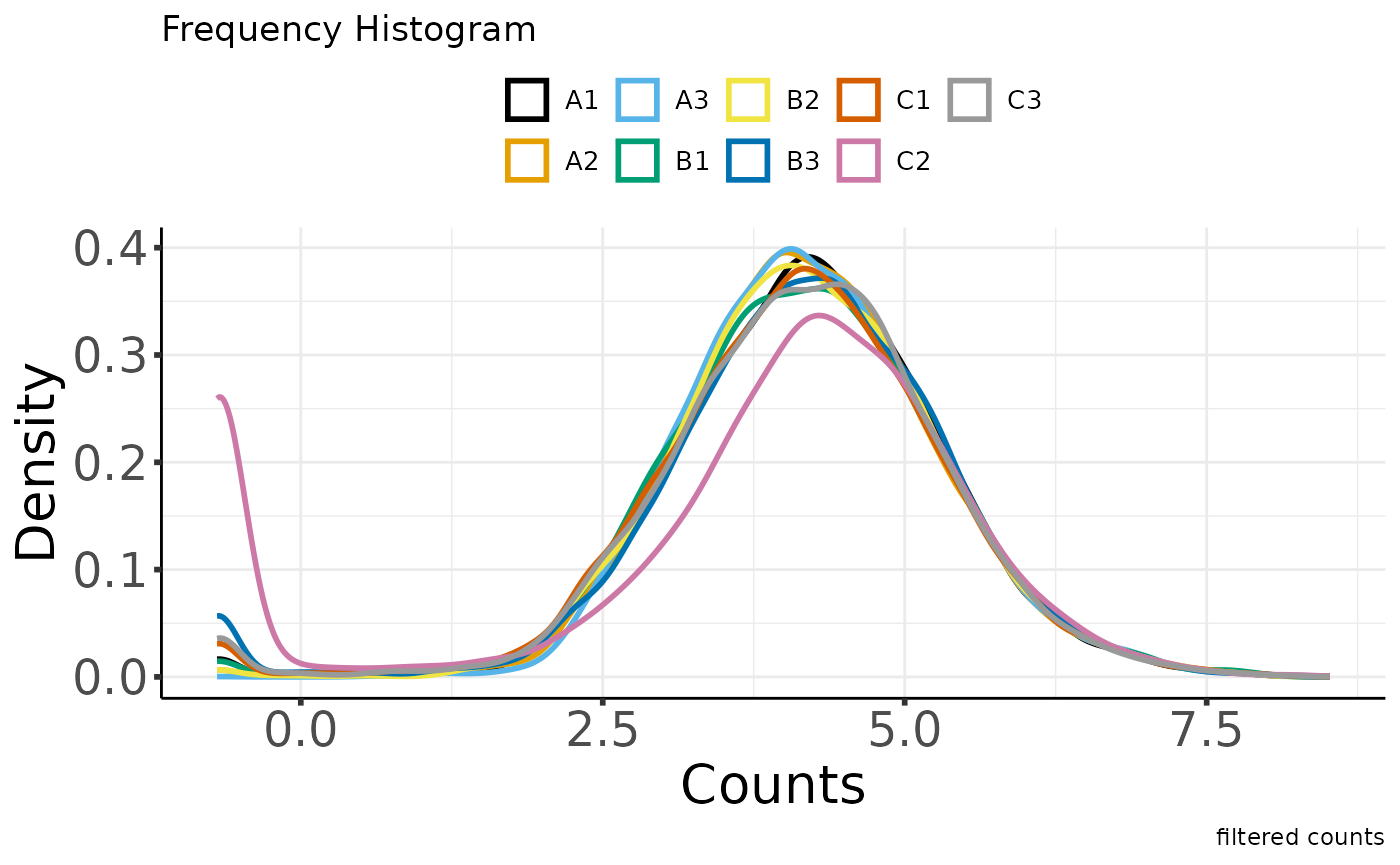
#> * filtering clean counts
#>
#> Number of features after filtering: 7943
#>
#> colors_for_plots NULL
#>
#> colors_for_plots character
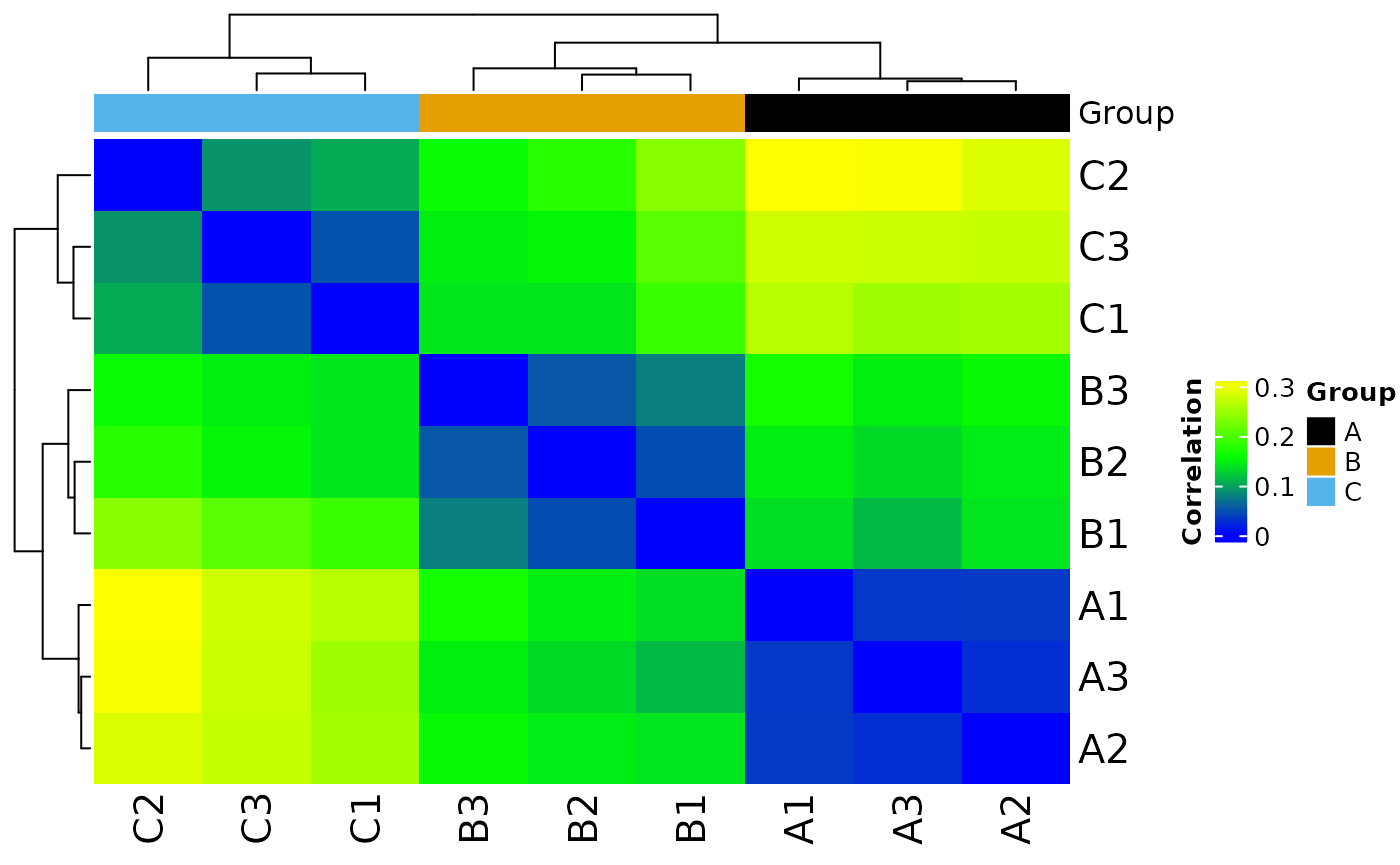
#> Saving 7.29 x 4.51 in image
#> Saving 7.29 x 4.51 in image
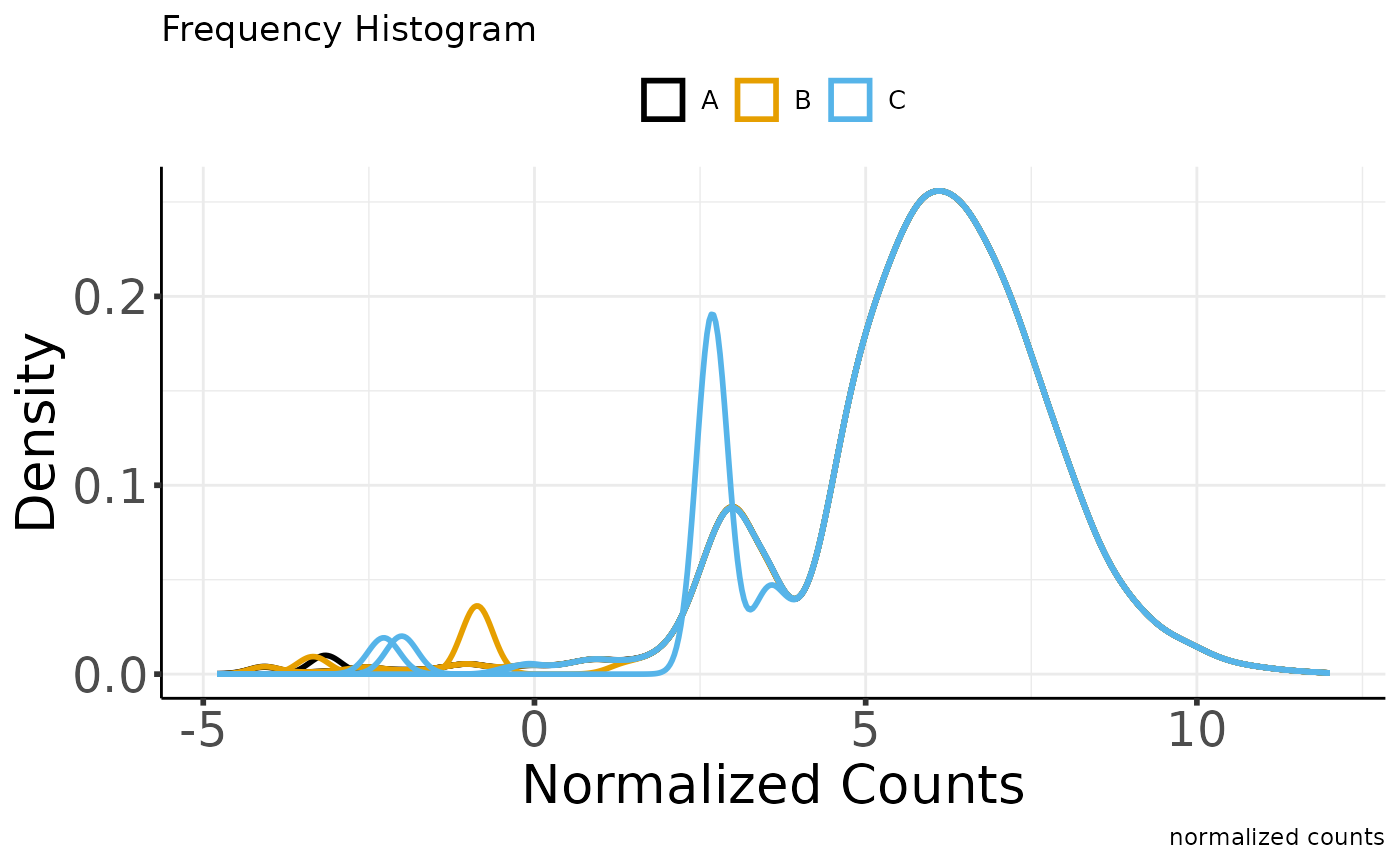
#> * normalizing filt counts
#>
#> Total number of features included: 7943
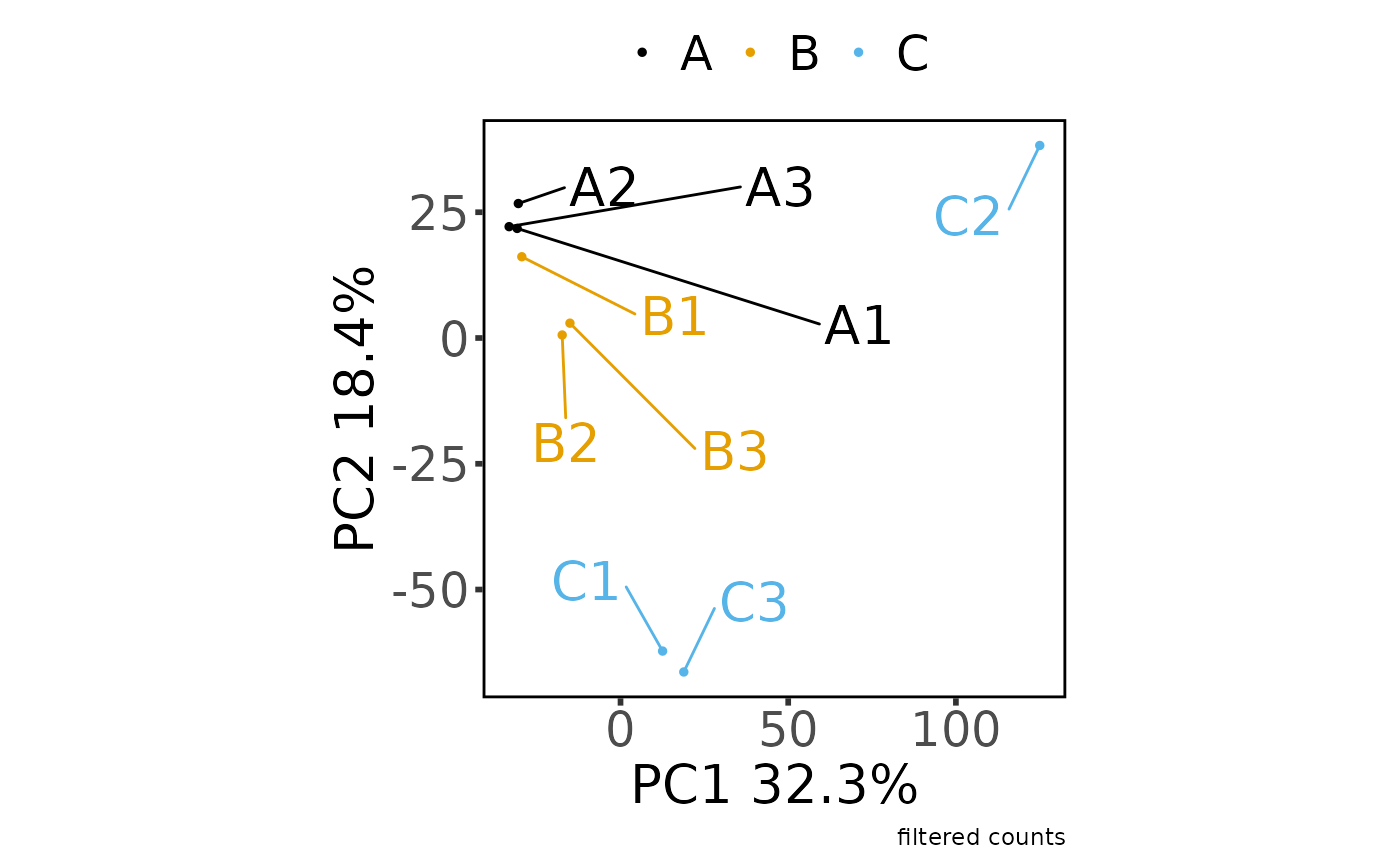
#> Warning: ggrepel: 1 unlabeled data points (too many overlaps). Consider
#> increasing max.overlaps
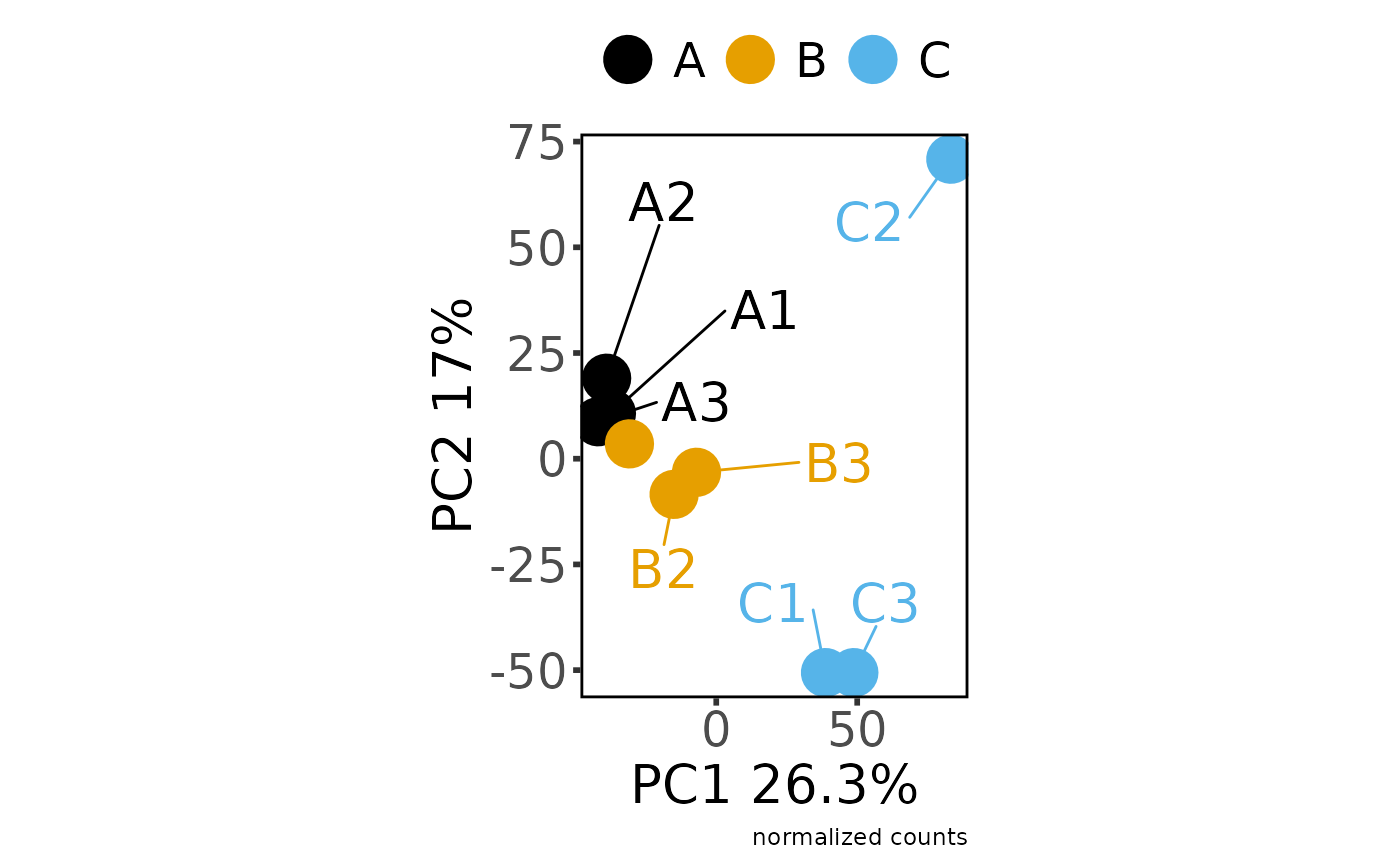
#> Warning: ggrepel: 1 unlabeled data points (too many overlaps). Consider
#> increasing max.overlaps
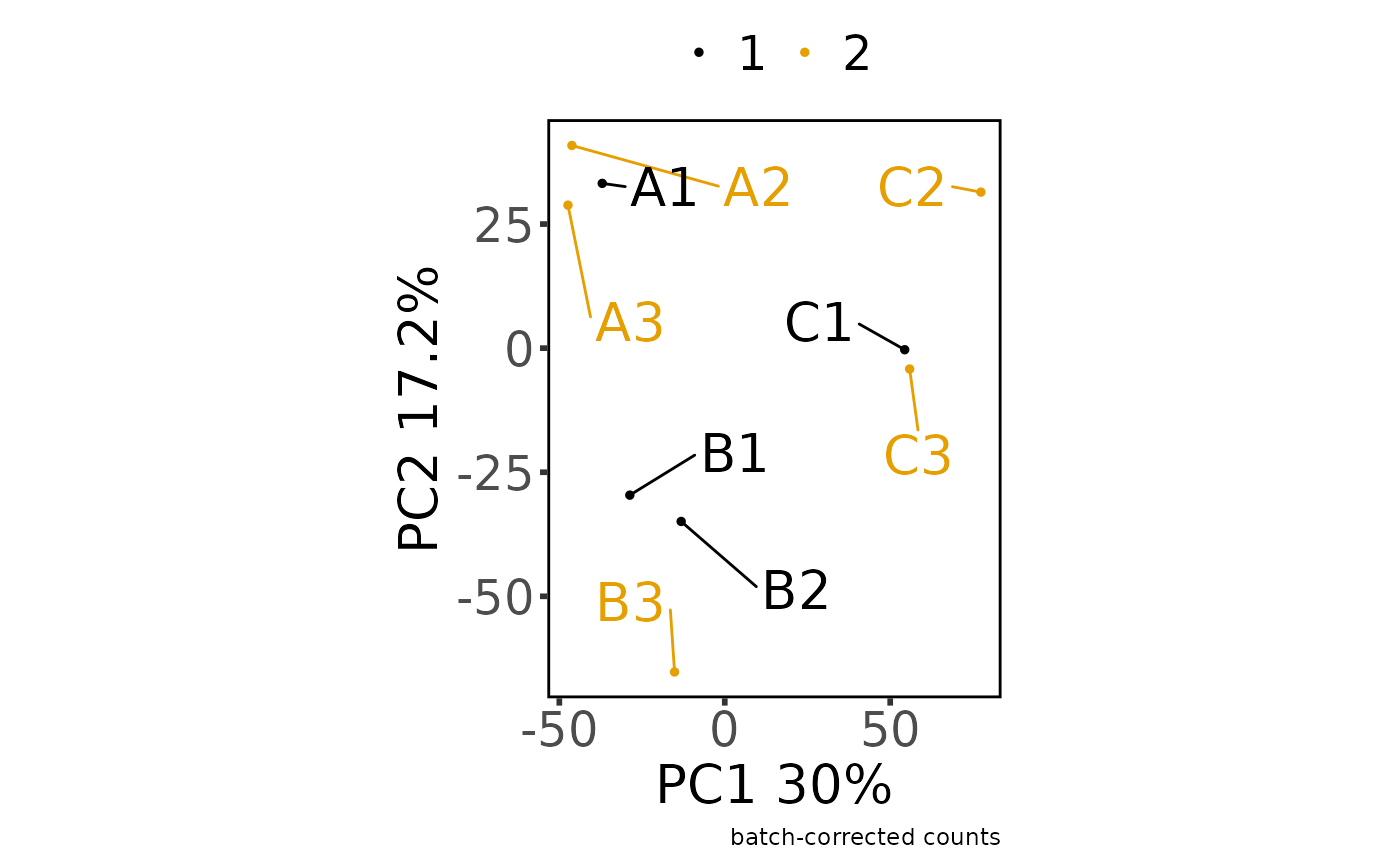
#> Saving 7.29 x 4.51 in image
#> Warning: ggrepel: 1 unlabeled data points (too many overlaps). Consider
#> increasing max.overlaps
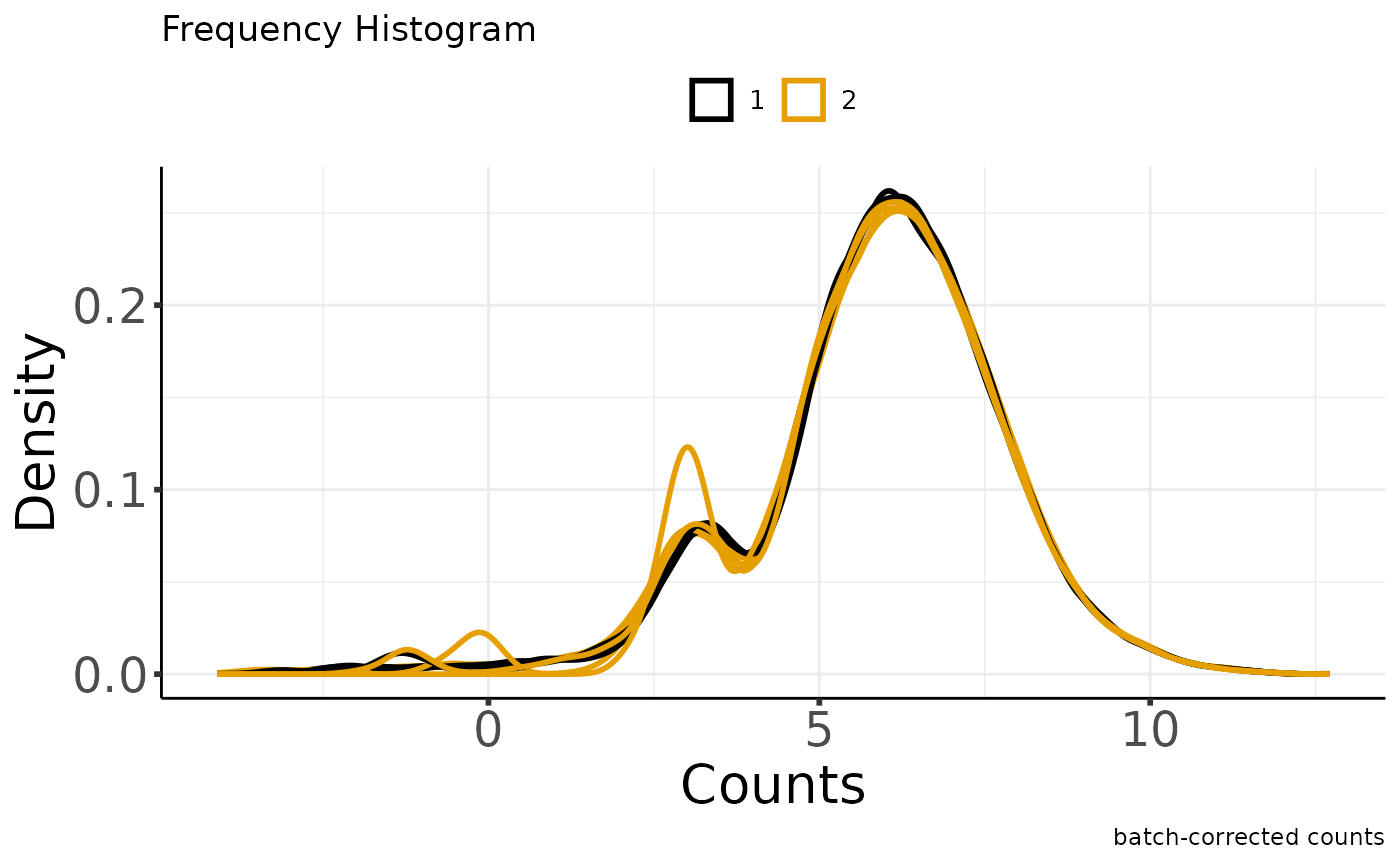
#> Saving 7.29 x 4.51 in image
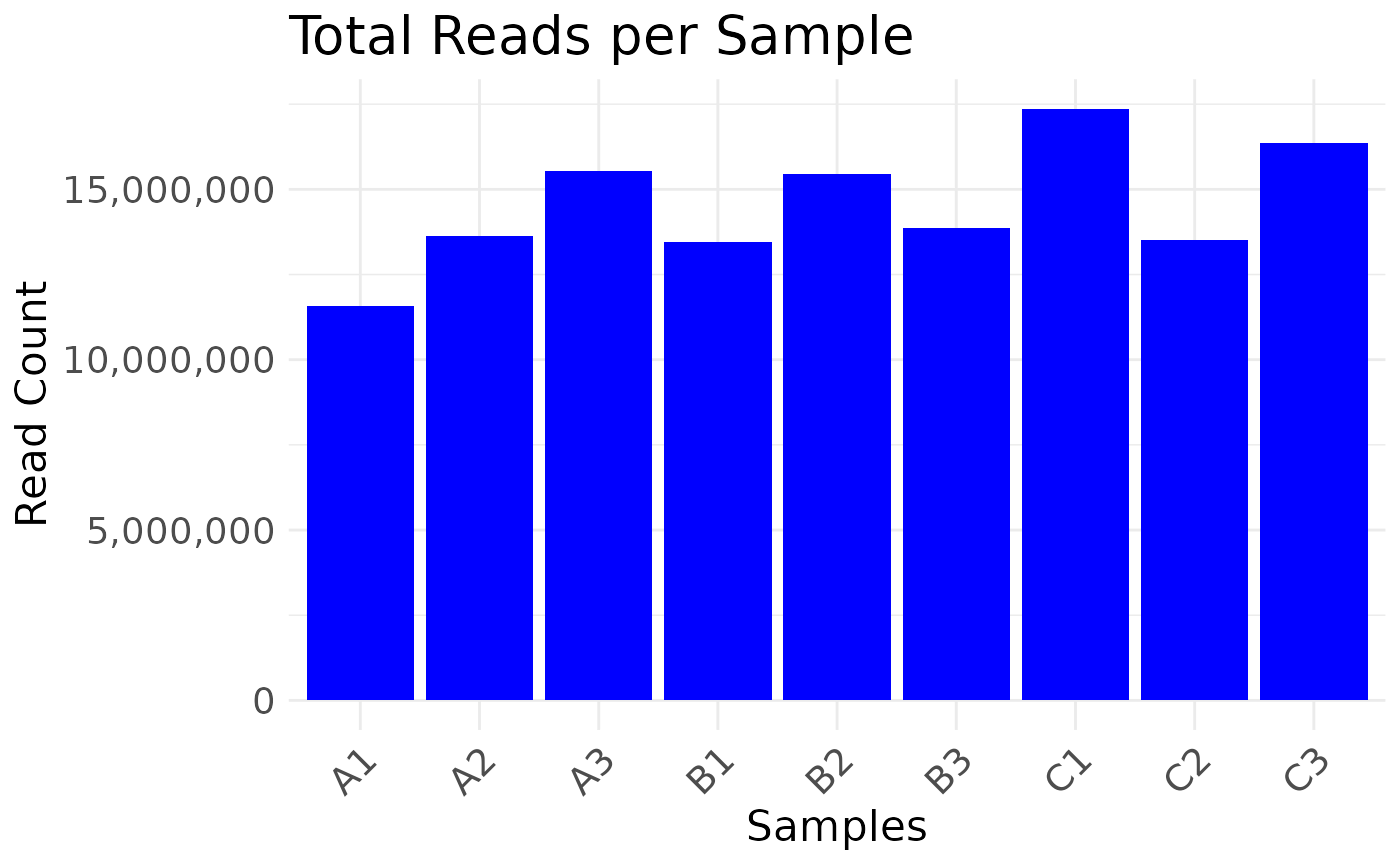
#> Sample columns: A1, Sample columns: A2, Sample columns: A3, Sample columns: B1, Sample columns: B2, Sample columns: B3, Sample columns: C1, Sample columns: C2, Sample columns: C3
#>
#> * batch-correcting norm-voom counts
#>
#> Found2batches
#>
#> Adjusting for2covariate(s) or covariate level(s)
#>
#> Standardizing Data across genes
#>
#> Fitting L/S model and finding priors
#>
#> Finding parametric adjustments
#>
#> Adjusting the Data
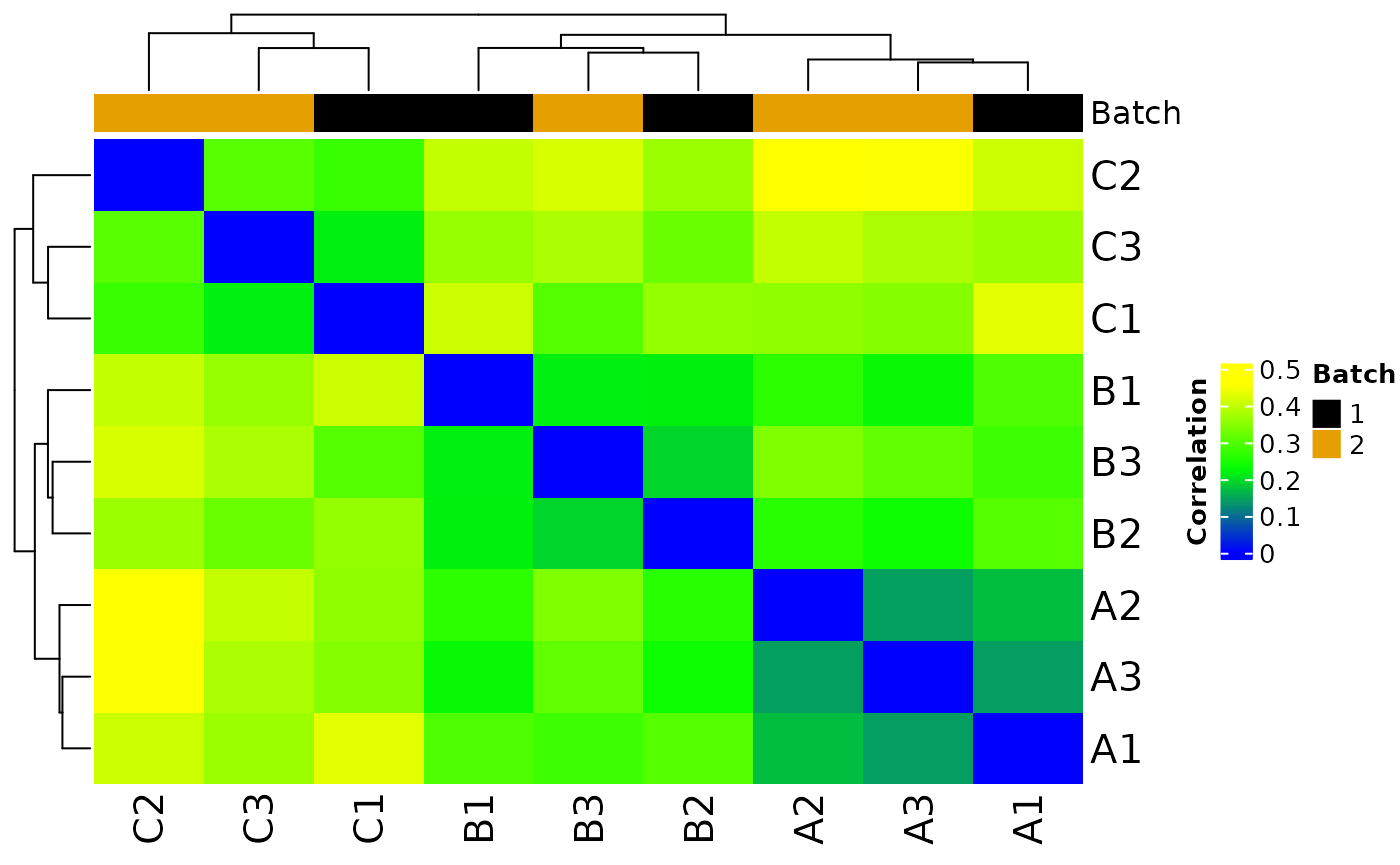
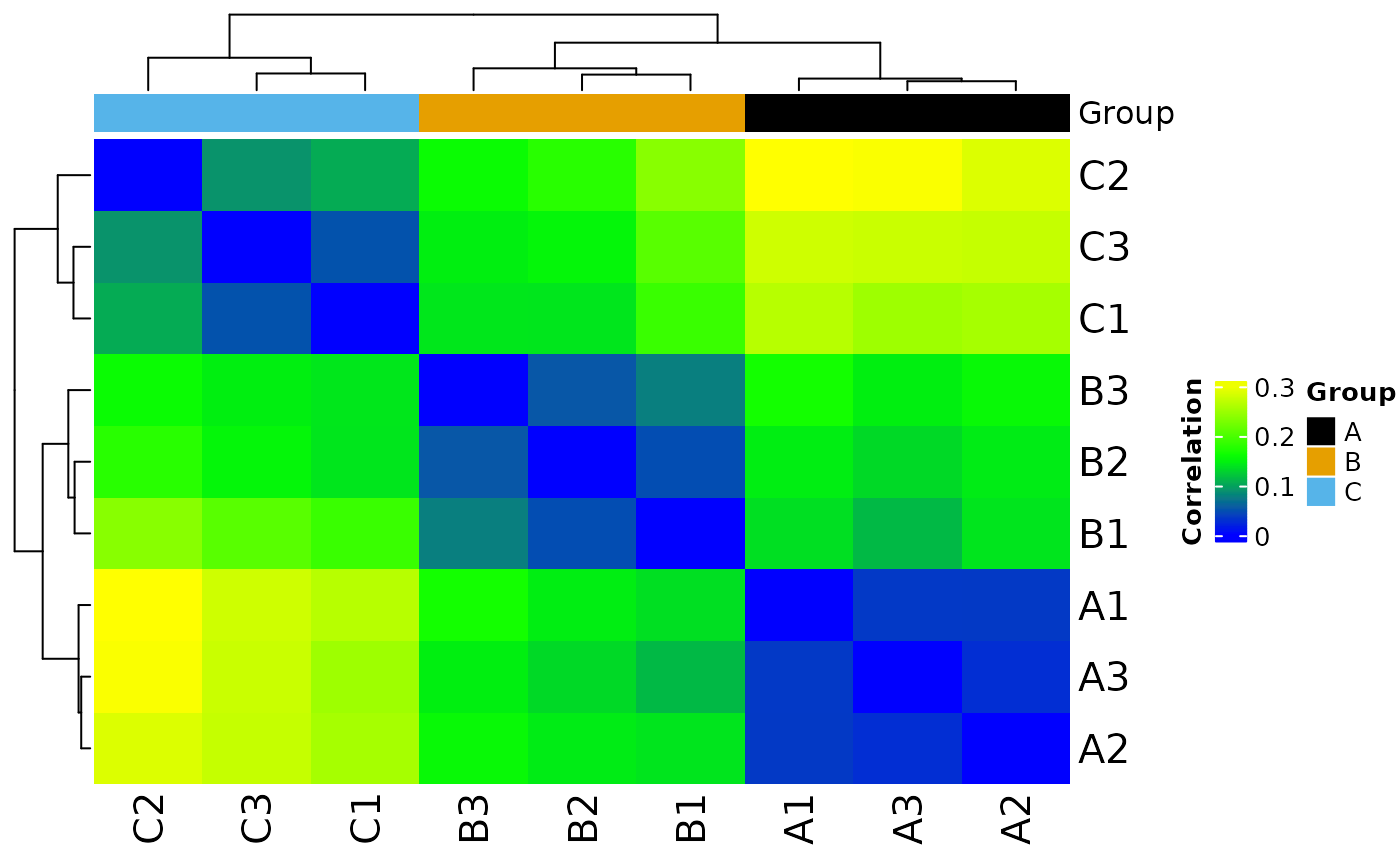
#> Saving 7.29 x 4.51 in image
#> Saving 7.29 x 4.51 in image
#> The total number of features in output: 7943
#> Number of samples after batch correction: 10
#> * differential counts
#> Setting first column of `counts` as gene annotation.
#> Total number of genes included: 7943
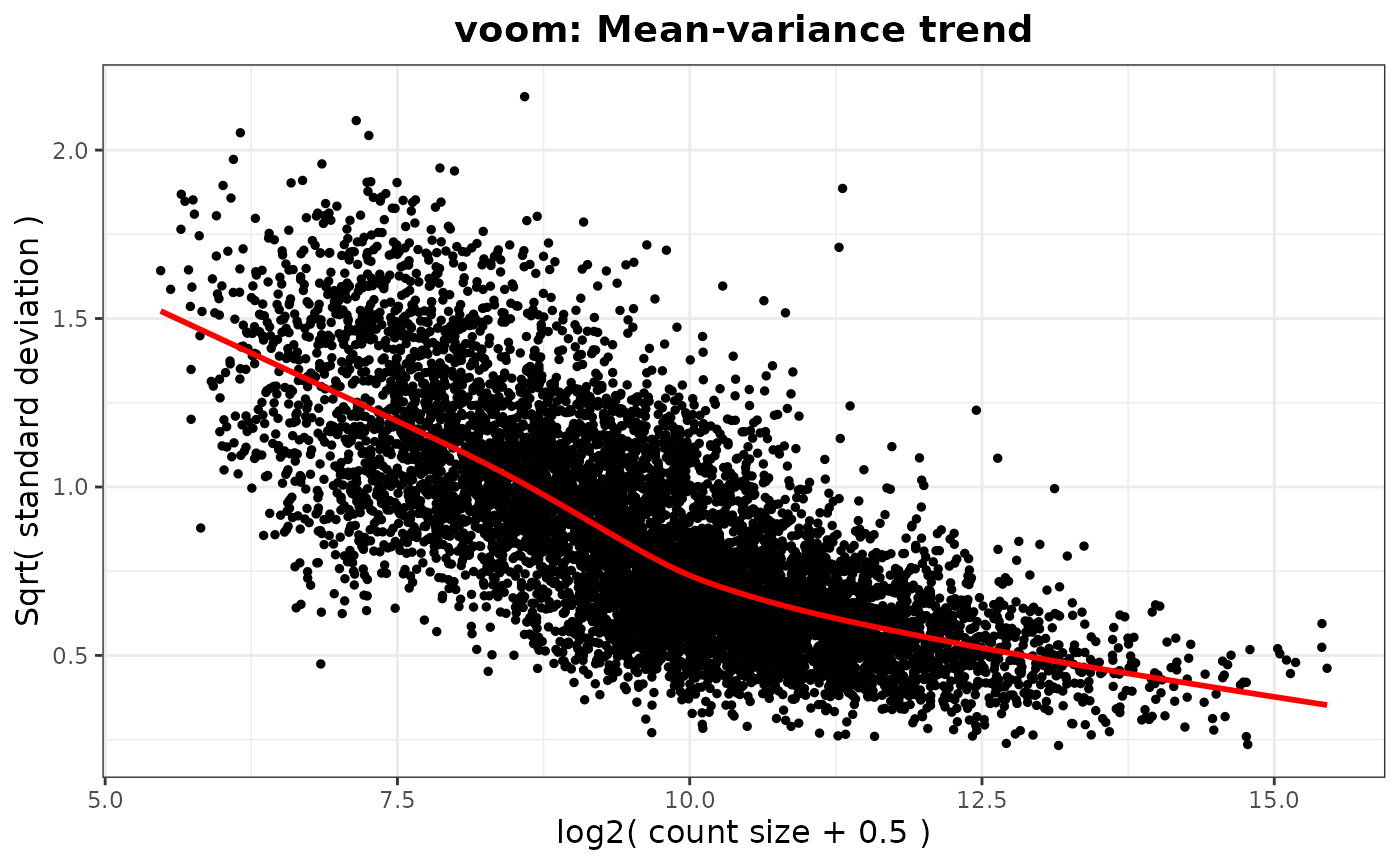
#> `geom_smooth()` using method = 'gam' and formula = 'y ~ s(x, bs = "cs")'
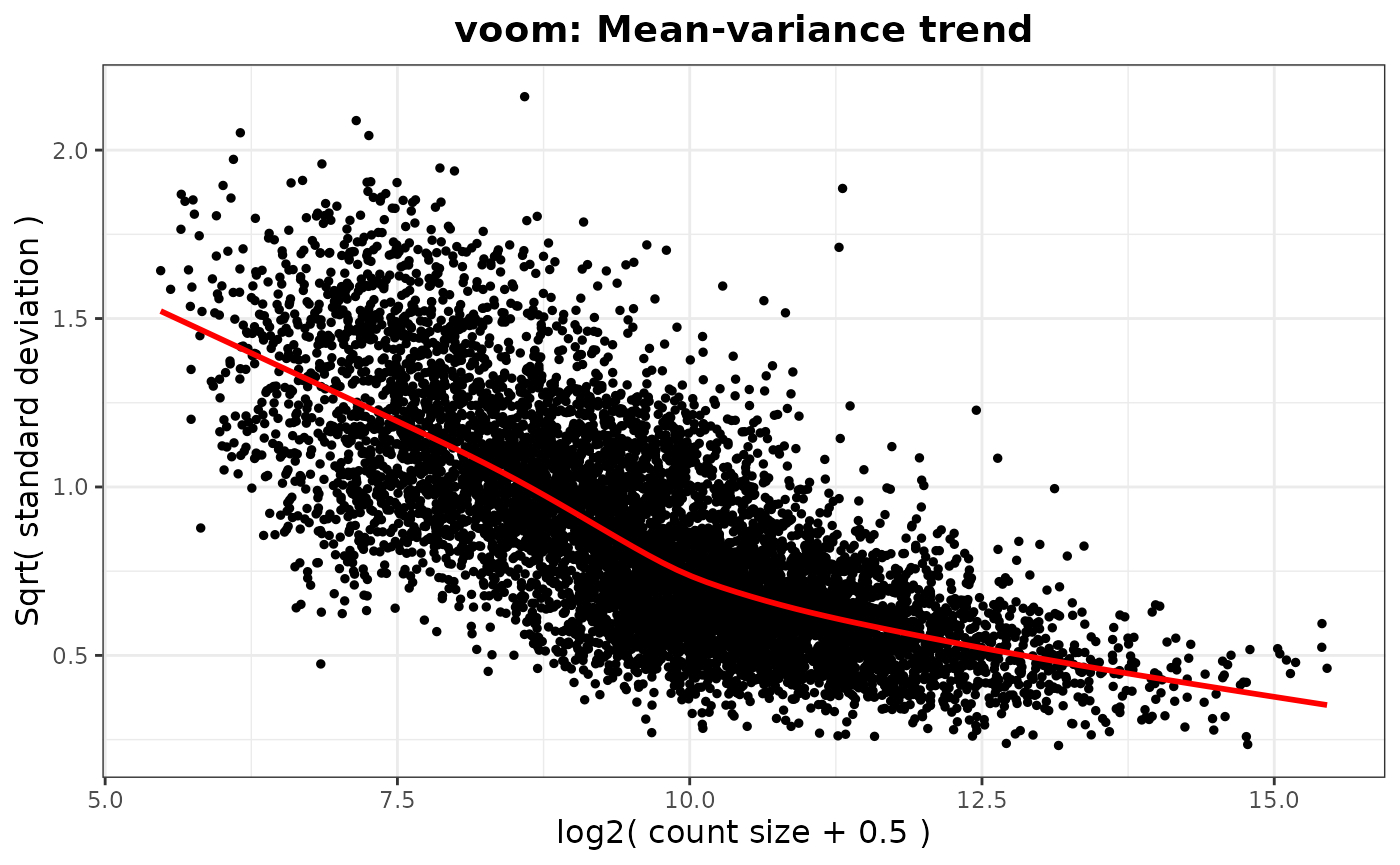
#> Saving 7.29 x 4.51 in image
#> `geom_smooth()` using method = 'gam' and formula = 'y ~ s(x, bs = "cs")'
#> Joining with `by = join_by(GeneName)`
#> Joining with `by = join_by(GeneName)`
#> * filtering differential features
#>
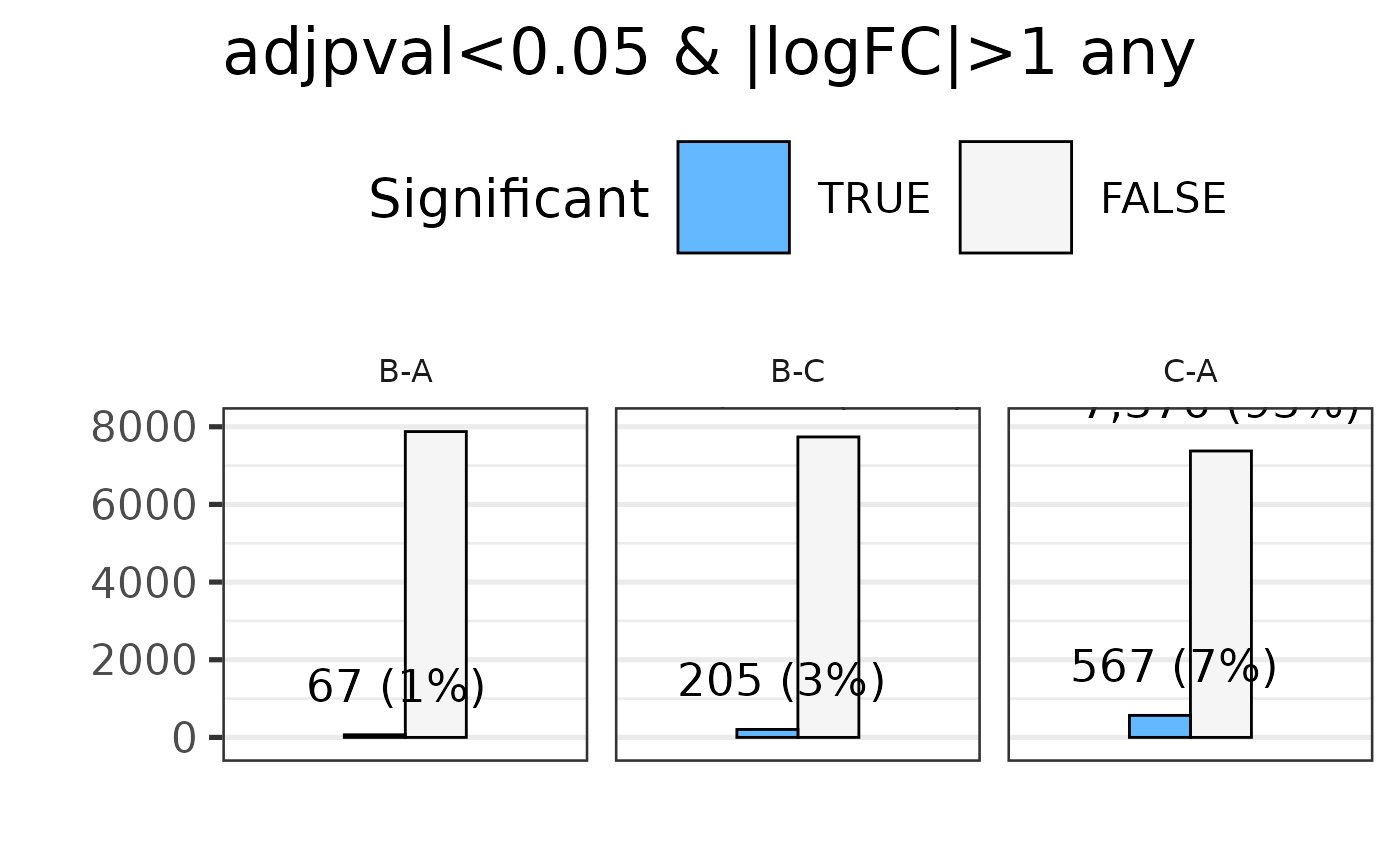
#> Total number of genes selected with adjpval < 0.05 and | logFC | ≥ 1 is sum(selgenes)
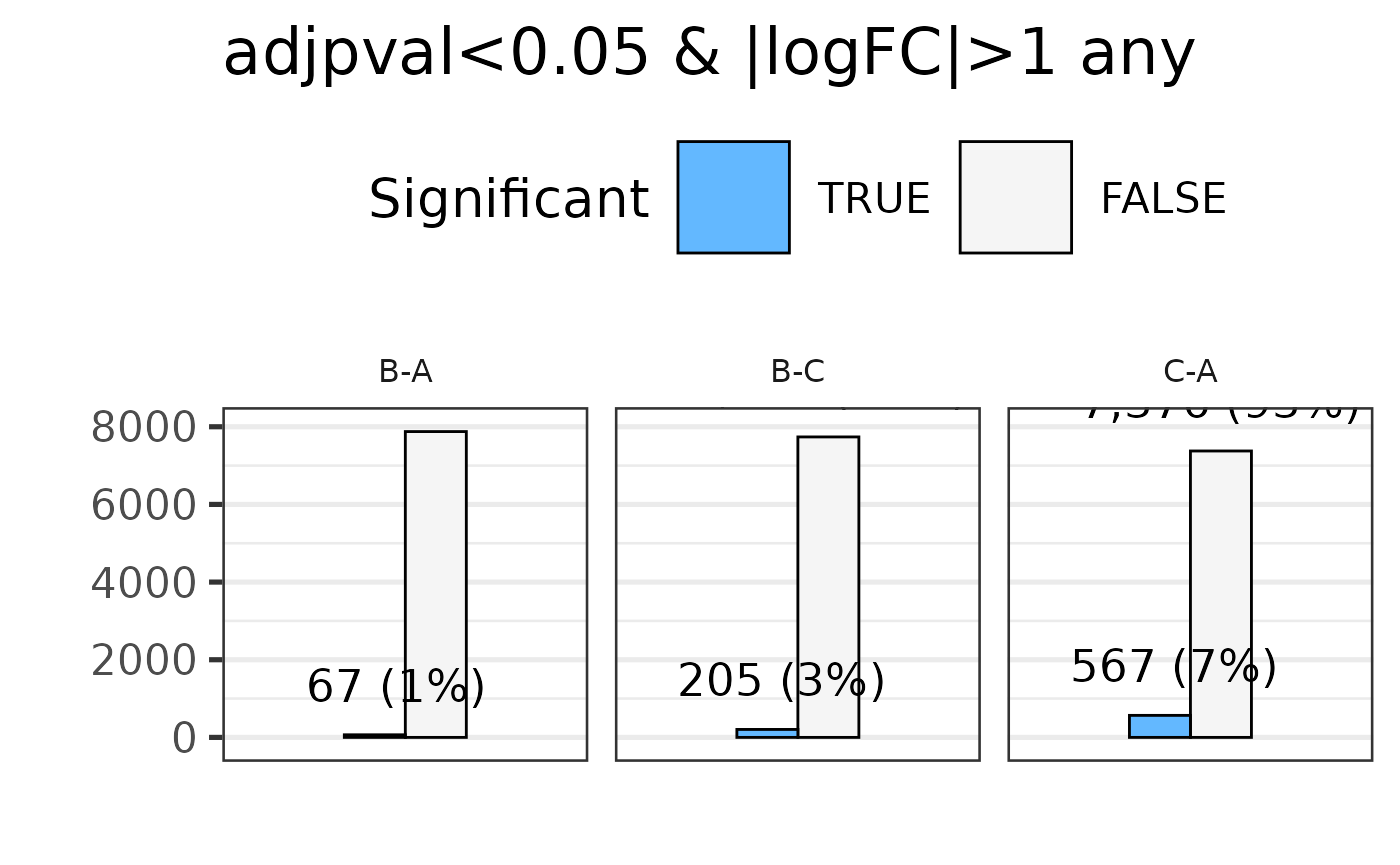
#> Saving 7.29 x 4.51 in image
moo_nidap@analyses$diff |>
join_dfs_wide() |>
head()
#> Joining with `by = join_by(GeneName)`
#> Joining with `by = join_by(GeneName)`
#> GeneName B-A_FC B-A_logFC B-A_tstat B-A_pval B-A_adjpval C-A_FC
#> 1 Mrpl15 1.059250 0.08304265 0.2377167 0.8162052 0.9682636 -1.068725
#> 2 Lypla1 1.370909 0.45513310 1.1301522 0.2810081 0.7797612 -1.066981
#> 3 Tcea1 1.083699 0.11596469 0.3657617 0.7210585 0.9500109 -1.177051
#> 4 Atp6v1h 1.311199 0.39088683 1.1241780 0.2834330 0.7825194 -1.221374
#> 5 Rb1cc1 1.514888 0.59921070 1.3095182 0.2154459 0.7187843 1.313927
#> 6 Pcmtd1 1.112738 0.15411405 0.2497788 0.8070821 0.9663212 3.238362
#> C-A_logFC C-A_tstat C-A_pval C-A_adjpval B-C_FC B-C_logFC
#> 1 -0.09589040 -0.2897955 0.777035116 0.89204033 1.132046 0.1789331
#> 2 -0.09353458 -0.2379598 0.816021040 0.91228196 1.462734 0.5486677
#> 3 -0.23517638 -0.7602905 0.462100568 0.69160897 1.275569 0.3511411
#> 4 -0.28850521 -0.8324070 0.421815393 0.65837683 1.601465 0.6793920
#> 5 0.39388567 0.9073581 0.382492765 0.62547476 1.152946 0.2053250
#> 6 1.69526432 3.4021010 0.005417489 0.05722644 -2.910264 -1.5411503
#> B-C_tstat B-C_pval B-C_adjpval
#> 1 0.5285947 0.60695058 0.8355016
#> 2 1.3910079 0.19006353 0.4871490
#> 3 1.1281746 0.28180907 0.5893629
#> 4 1.9865449 0.07085778 0.3168579
#> 5 0.4843119 0.63708764 0.8518020
#> 6 -2.9536960 0.01233497 0.1411803
moo_nidap@analyses$diff_filt |> head()
#> GeneName B-A_FC B-A_logFC B-A_tstat B-A_pval B-A_adjpval C-A_FC C-A_logFC
#> 1 Rrs1 -2.06 -1.040 -2.860 0.0147 0.276 -2.71 -1.44
#> 2 Mcm3 -1.45 -0.539 -1.870 0.0869 0.544 -2.45 -1.29
#> 3 Ogfrl1 1.07 0.102 0.292 0.7750 0.960 -3.78 -1.92
#> 4 Smap1 2.96 1.570 2.010 0.0684 0.499 5.68 2.51
#> 5 Plekhb2 -1.24 -0.312 -1.100 0.2950 0.789 2.69 1.43
#> 6 Il18r1 2.41 1.270 0.706 0.4940 0.875 36.40 5.19
#> C-A_tstat C-A_pval C-A_adjpval B-C_FC B-C_logFC B-C_tstat B-C_pval
#> 1 -3.83 2.48e-03 0.03680 1.32 0.399 0.941 3.66e-01
#> 2 -4.33 1.03e-03 0.02240 1.69 0.755 2.380 3.53e-02
#> 3 -4.04 1.71e-03 0.03030 4.05 2.020 4.030 1.76e-03
#> 4 3.59 3.82e-03 0.04700 -1.92 -0.938 -1.750 1.07e-01
#> 5 5.99 6.94e-05 0.00463 -3.34 -1.740 -6.980 1.66e-05
#> 6 3.13 8.86e-03 0.07640 -15.10 -3.920 -4.230 1.23e-03
#> B-C_adjpval
#> 1 0.6660
#> 2 0.2300
#> 3 0.0550
#> 4 0.3780
#> 5 0.0033
#> 6 0.0455
The multiOmicDataSet object structure
str(moo_nidap)
#> <MOSuite::multiOmicDataSet>
#> @ sample_meta:'data.frame': 9 obs. of 5 variables:
#> .. $ Sample : chr "A1" "A2" "A3" "B1" ...
#> .. $ Group : chr "A" "A" "A" "B" ...
#> .. $ Replicate: num 1 2 3 1 2 3 1 2 3
#> .. $ Batch : num 1 2 2 1 1 2 1 2 2
#> .. $ Label : chr "A1" "A2" "A3" "B1" ...
#> .. - attr(*, "spec")=List of 3
#> .. ..$ cols :List of 5
#> .. .. ..$ Sample : list()
#> .. .. .. ..- attr(*, "class")= chr [1:2] "collector_character" "collector"
#> .. .. ..$ Group : list()
#> .. .. .. ..- attr(*, "class")= chr [1:2] "collector_character" "collector"
#> .. .. ..$ Replicate: list()
#> .. .. .. ..- attr(*, "class")= chr [1:2] "collector_double" "collector"
#> .. .. ..$ Batch : list()
#> .. .. .. ..- attr(*, "class")= chr [1:2] "collector_double" "collector"
#> .. .. ..$ Label : list()
#> .. .. .. ..- attr(*, "class")= chr [1:2] "collector_character" "collector"
#> .. ..$ default: list()
#> .. .. ..- attr(*, "class")= chr [1:2] "collector_guess" "collector"
#> .. ..$ delim : chr ","
#> .. ..- attr(*, "class")= chr "col_spec"
#> .. - attr(*, "problems")=<externalptr>
#> @ annotation :'data.frame': 43280 obs. of 1 variable:
#> .. $ GeneName: chr "RP23-271O17.1" "Gm26206" "Xkr4" "RP23-317L18.1" ...
#> .. - attr(*, "spec")=List of 3
#> .. ..$ cols :List of 10
#> .. .. ..$ GeneName: list()
#> .. .. .. ..- attr(*, "class")= chr [1:2] "collector_character" "collector"
#> .. .. ..$ A1 : list()
#> .. .. .. ..- attr(*, "class")= chr [1:2] "collector_double" "collector"
#> .. .. ..$ A2 : list()
#> .. .. .. ..- attr(*, "class")= chr [1:2] "collector_double" "collector"
#> .. .. ..$ A3 : list()
#> .. .. .. ..- attr(*, "class")= chr [1:2] "collector_double" "collector"
#> .. .. ..$ B1 : list()
#> .. .. .. ..- attr(*, "class")= chr [1:2] "collector_double" "collector"
#> .. .. ..$ B2 : list()
#> .. .. .. ..- attr(*, "class")= chr [1:2] "collector_double" "collector"
#> .. .. ..$ B3 : list()
#> .. .. .. ..- attr(*, "class")= chr [1:2] "collector_double" "collector"
#> .. .. ..$ C1 : list()
#> .. .. .. ..- attr(*, "class")= chr [1:2] "collector_double" "collector"
#> .. .. ..$ C2 : list()
#> .. .. .. ..- attr(*, "class")= chr [1:2] "collector_double" "collector"
#> .. .. ..$ C3 : list()
#> .. .. .. ..- attr(*, "class")= chr [1:2] "collector_double" "collector"
#> .. ..$ default: list()
#> .. .. ..- attr(*, "class")= chr [1:2] "collector_guess" "collector"
#> .. ..$ delim : chr ","
#> .. ..- attr(*, "class")= chr "col_spec"
#> .. - attr(*, "problems")=<externalptr>
#> @ counts :List of 5
#> .. $ raw :'data.frame': 43280 obs. of 10 variables:
#> .. ..$ GeneName: chr [1:43280] "RP23-271O17.1" "Gm26206" "Xkr4" "RP23-317L18.1" ...
#> .. ..$ A1 : num [1:43280] 0 0 0 0 0 0 0 0 0 0 ...
#> .. ..$ A2 : num [1:43280] 0 0 0 0 0 0 0 0 0 0 ...
#> .. ..$ A3 : num [1:43280] 0 0 0 0 0 0 0 0 0 0 ...
#> .. ..$ B1 : num [1:43280] 0 0 0 0 0 0 0 0 0 0 ...
#> .. ..$ B2 : num [1:43280] 0 0 0 0 0 0 0 0 0 1 ...
#> .. ..$ B3 : num [1:43280] 0 0 0 0 0 0 0 0 0 0 ...
#> .. ..$ C1 : num [1:43280] 0 0 0 0 0 0 0 0 0 0 ...
#> .. ..$ C2 : num [1:43280] 0 0 0 0 0 0 0 0 0 0 ...
#> .. ..$ C3 : num [1:43280] 0 0 0 0 0 0 0 0 0 0 ...
#> .. ..- attr(*, "spec")=List of 3
#> .. .. ..$ cols :List of 10
#> .. .. .. ..$ GeneName: list()
#> .. .. .. .. ..- attr(*, "class")= chr [1:2] "collector_character" "collector"
#> .. .. .. ..$ A1 : list()
#> .. .. .. .. ..- attr(*, "class")= chr [1:2] "collector_double" "collector"
#> .. .. .. ..$ A2 : list()
#> .. .. .. .. ..- attr(*, "class")= chr [1:2] "collector_double" "collector"
#> .. .. .. ..$ A3 : list()
#> .. .. .. .. ..- attr(*, "class")= chr [1:2] "collector_double" "collector"
#> .. .. .. ..$ B1 : list()
#> .. .. .. .. ..- attr(*, "class")= chr [1:2] "collector_double" "collector"
#> .. .. .. ..$ B2 : list()
#> .. .. .. .. ..- attr(*, "class")= chr [1:2] "collector_double" "collector"
#> .. .. .. ..$ B3 : list()
#> .. .. .. .. ..- attr(*, "class")= chr [1:2] "collector_double" "collector"
#> .. .. .. ..$ C1 : list()
#> .. .. .. .. ..- attr(*, "class")= chr [1:2] "collector_double" "collector"
#> .. .. .. ..$ C2 : list()
#> .. .. .. .. ..- attr(*, "class")= chr [1:2] "collector_double" "collector"
#> .. .. .. ..$ C3 : list()
#> .. .. .. .. ..- attr(*, "class")= chr [1:2] "collector_double" "collector"
#> .. .. ..$ default: list()
#> .. .. .. ..- attr(*, "class")= chr [1:2] "collector_guess" "collector"
#> .. .. ..$ delim : chr ","
#> .. .. ..- attr(*, "class")= chr "col_spec"
#> .. ..- attr(*, "problems")=<externalptr>
#> .. $ clean:'data.frame': 43280 obs. of 10 variables:
#> .. ..$ GeneName: chr [1:43280] "RP23-271O17.1" "Gm26206" "Xkr4" "RP23-317L18.1" ...
#> .. ..$ A1 : num [1:43280] 0 0 0 0 0 0 0 0 0 0 ...
#> .. ..$ A2 : num [1:43280] 0 0 0 0 0 0 0 0 0 0 ...
#> .. ..$ A3 : num [1:43280] 0 0 0 0 0 0 0 0 0 0 ...
#> .. ..$ B1 : num [1:43280] 0 0 0 0 0 0 0 0 0 0 ...
#> .. ..$ B2 : num [1:43280] 0 0 0 0 0 0 0 0 0 1 ...
#> .. ..$ B3 : num [1:43280] 0 0 0 0 0 0 0 0 0 0 ...
#> .. ..$ C1 : num [1:43280] 0 0 0 0 0 0 0 0 0 0 ...
#> .. ..$ C2 : num [1:43280] 0 0 0 0 0 0 0 0 0 0 ...
#> .. ..$ C3 : num [1:43280] 0 0 0 0 0 0 0 0 0 0 ...
#> .. ..- attr(*, "spec")=List of 3
#> .. .. ..$ cols :List of 10
#> .. .. .. ..$ GeneName: list()
#> .. .. .. .. ..- attr(*, "class")= chr [1:2] "collector_character" "collector"
#> .. .. .. ..$ A1 : list()
#> .. .. .. .. ..- attr(*, "class")= chr [1:2] "collector_double" "collector"
#> .. .. .. ..$ A2 : list()
#> .. .. .. .. ..- attr(*, "class")= chr [1:2] "collector_double" "collector"
#> .. .. .. ..$ A3 : list()
#> .. .. .. .. ..- attr(*, "class")= chr [1:2] "collector_double" "collector"
#> .. .. .. ..$ B1 : list()
#> .. .. .. .. ..- attr(*, "class")= chr [1:2] "collector_double" "collector"
#> .. .. .. ..$ B2 : list()
#> .. .. .. .. ..- attr(*, "class")= chr [1:2] "collector_double" "collector"
#> .. .. .. ..$ B3 : list()
#> .. .. .. .. ..- attr(*, "class")= chr [1:2] "collector_double" "collector"
#> .. .. .. ..$ C1 : list()
#> .. .. .. .. ..- attr(*, "class")= chr [1:2] "collector_double" "collector"
#> .. .. .. ..$ C2 : list()
#> .. .. .. .. ..- attr(*, "class")= chr [1:2] "collector_double" "collector"
#> .. .. .. ..$ C3 : list()
#> .. .. .. .. ..- attr(*, "class")= chr [1:2] "collector_double" "collector"
#> .. .. ..$ default: list()
#> .. .. .. ..- attr(*, "class")= chr [1:2] "collector_guess" "collector"
#> .. .. ..$ delim : chr ","
#> .. .. ..- attr(*, "class")= chr "col_spec"
#> .. ..- attr(*, "problems")=<externalptr>
#> .. $ filt :'data.frame': 7943 obs. of 10 variables:
#> .. ..$ GeneName: chr [1:7943] "Mrpl15" "Lypla1" "Tcea1" "Atp6v1h" ...
#> .. ..$ A1 : num [1:7943] 1245 1483 1381 1033 666 ...
#> .. ..$ A2 : num [1:7943] 1341 1410 2044 1959 1397 ...
#> .. ..$ A3 : num [1:7943] 1476 1370 2051 1890 1576 ...
#> .. ..$ B1 : num [1:7943] 965 1146 2325 2075 681 ...
#> .. ..$ B2 : num [1:7943] 1235 1422 2386 2702 2040 ...
#> .. ..$ B3 : num [1:7943] 1784 2624 1893 2150 1988 ...
#> .. ..$ C1 : num [1:7943] 1058 991 2391 2436 774 ...
#> .. ..$ C2 : num [1:7943] 1732 1101 916 1321 1921 ...
#> .. ..$ C3 : num [1:7943] 1531 2352 2261 1018 2660 ...
#> .. ..- attr(*, "spec")=List of 3
#> .. .. ..$ cols :List of 10
#> .. .. .. ..$ GeneName: list()
#> .. .. .. .. ..- attr(*, "class")= chr [1:2] "collector_character" "collector"
#> .. .. .. ..$ A1 : list()
#> .. .. .. .. ..- attr(*, "class")= chr [1:2] "collector_double" "collector"
#> .. .. .. ..$ A2 : list()
#> .. .. .. .. ..- attr(*, "class")= chr [1:2] "collector_double" "collector"
#> .. .. .. ..$ A3 : list()
#> .. .. .. .. ..- attr(*, "class")= chr [1:2] "collector_double" "collector"
#> .. .. .. ..$ B1 : list()
#> .. .. .. .. ..- attr(*, "class")= chr [1:2] "collector_double" "collector"
#> .. .. .. ..$ B2 : list()
#> .. .. .. .. ..- attr(*, "class")= chr [1:2] "collector_double" "collector"
#> .. .. .. ..$ B3 : list()
#> .. .. .. .. ..- attr(*, "class")= chr [1:2] "collector_double" "collector"
#> .. .. .. ..$ C1 : list()
#> .. .. .. .. ..- attr(*, "class")= chr [1:2] "collector_double" "collector"
#> .. .. .. ..$ C2 : list()
#> .. .. .. .. ..- attr(*, "class")= chr [1:2] "collector_double" "collector"
#> .. .. .. ..$ C3 : list()
#> .. .. .. .. ..- attr(*, "class")= chr [1:2] "collector_double" "collector"
#> .. .. ..$ default: list()
#> .. .. .. ..- attr(*, "class")= chr [1:2] "collector_guess" "collector"
#> .. .. ..$ delim : chr ","
#> .. .. ..- attr(*, "class")= chr "col_spec"
#> .. ..- attr(*, "problems")=<externalptr>
#> .. $ norm :List of 1
#> .. ..$ voom:'data.frame': 7943 obs. of 10 variables:
#> .. .. ..$ GeneName: chr [1:7943] "Mrpl15" "Lypla1" "Tcea1" "Atp6v1h" ...
#> .. .. ..$ A1 : num [1:7943] 6.79 7.04 6.93 6.51 5.85 ...
#> .. .. ..$ A2 : num [1:7943] 6.7 6.78 7.32 7.26 6.76 ...
#> .. .. ..$ A3 : num [1:7943] 6.64 6.53 7.13 7 6.75 ...
#> .. .. ..$ B1 : num [1:7943] 6.22 6.47 7.48 7.31 5.75 ...
#> .. .. ..$ B2 : num [1:7943] 6.38 6.57 7.33 7.52 7.11 ...
#> .. .. ..$ B3 : num [1:7943] 7.02 7.59 7.11 7.3 7.17 ...
#> .. .. ..$ C1 : num [1:7943] 6.02 5.92 7.19 7.22 5.56 ...
#> .. .. ..$ C2 : num [1:7943] 7.03 6.43 6.19 6.67 7.19 ...
#> .. .. ..$ C3 : num [1:7943] 6.6 7.25 7.2 6.01 7.43 ...
#> .. $ batch:'data.frame': 7943 obs. of 10 variables:
#> .. ..$ GeneName: chr [1:7943] "Mrpl15" "Lypla1" "Tcea1" "Atp6v1h" ...
#> .. ..$ A1 : num [1:7943] 6.92 7.2 6.87 6.51 6.23 ...
#> .. ..$ A2 : num [1:7943] 6.59 6.66 7.33 7.25 6.46 ...
#> .. ..$ A3 : num [1:7943] 6.54 6.43 7.16 7.02 6.45 ...
#> .. ..$ B1 : num [1:7943] 6.41 6.68 7.42 7.28 6.27 ...
#> .. ..$ B2 : num [1:7943] 6.55 6.78 7.27 7.47 7.35 ...
#> .. ..$ B3 : num [1:7943] 6.88 7.4 7.16 7.33 6.89 ...
#> .. ..$ C1 : num [1:7943] 6.21 6.13 7.13 7.13 6.06 ...
#> .. ..$ C2 : num [1:7943] 6.87 6.32 6.32 6.69 6.88 ...
#> .. ..$ C3 : num [1:7943] 6.48 7.04 7.18 6.1 7.1 ...
#> @ analyses :List of 3
#> .. $ colors :List of 5
#> .. ..$ Sample : Named chr [1:9] "#000000" "#E69F00" "#56B4E9" "#009E73" ...
#> .. .. ..- attr(*, "names")= chr [1:9] "A1" "A2" "A3" "B1" ...
#> .. ..$ Group : Named chr [1:3] "#000000" "#E69F00" "#56B4E9"
#> .. .. ..- attr(*, "names")= chr [1:3] "A" "B" "C"
#> .. ..$ Replicate: Named chr [1:3] "#000000" "#E69F00" "#56B4E9"
#> .. .. ..- attr(*, "names")= chr [1:3] "1" "2" "3"
#> .. ..$ Batch : Named chr [1:2] "#000000" "#E69F00"
#> .. .. ..- attr(*, "names")= chr [1:2] "1" "2"
#> .. ..$ Label : Named chr [1:9] "#000000" "#E69F00" "#56B4E9" "#009E73" ...
#> .. .. ..- attr(*, "names")= chr [1:9] "A1" "A2" "A3" "B1" ...
#> .. $ diff :List of 3
#> .. ..$ B-A:'data.frame': 7943 obs. of 6 variables:
#> .. .. ..$ GeneName: chr [1:7943] "Mrpl15" "Lypla1" "Tcea1" "Atp6v1h" ...
#> .. .. ..$ FC : num [1:7943] 1.06 1.37 1.08 1.31 1.51 ...
#> .. .. ..$ logFC : num [1:7943] 0.083 0.455 0.116 0.391 0.599 ...
#> .. .. ..$ tstat : num [1:7943] 0.238 1.13 0.366 1.124 1.31 ...
#> .. .. ..$ pval : num [1:7943] 0.816 0.281 0.721 0.283 0.215 ...
#> .. .. ..$ adjpval : num [1:7943] 0.968 0.78 0.95 0.783 0.719 ...
#> .. ..$ C-A:'data.frame': 7943 obs. of 6 variables:
#> .. .. ..$ GeneName: chr [1:7943] "Mrpl15" "Lypla1" "Tcea1" "Atp6v1h" ...
#> .. .. ..$ FC : num [1:7943] -1.07 -1.07 -1.18 -1.22 1.31 ...
#> .. .. ..$ logFC : num [1:7943] -0.0959 -0.0935 -0.2352 -0.2885 0.3939 ...
#> .. .. ..$ tstat : num [1:7943] -0.29 -0.238 -0.76 -0.832 0.907 ...
#> .. .. ..$ pval : num [1:7943] 0.777 0.816 0.462 0.422 0.382 ...
#> .. .. ..$ adjpval : num [1:7943] 0.892 0.912 0.692 0.658 0.625 ...
#> .. ..$ B-C:'data.frame': 7943 obs. of 6 variables:
#> .. .. ..$ GeneName: chr [1:7943] "Mrpl15" "Lypla1" "Tcea1" "Atp6v1h" ...
#> .. .. ..$ FC : num [1:7943] 1.13 1.46 1.28 1.6 1.15 ...
#> .. .. ..$ logFC : num [1:7943] 0.179 0.549 0.351 0.679 0.205 ...
#> .. .. ..$ tstat : num [1:7943] 0.529 1.391 1.128 1.987 0.484 ...
#> .. .. ..$ pval : num [1:7943] 0.607 0.1901 0.2818 0.0709 0.6371 ...
#> .. .. ..$ adjpval : num [1:7943] 0.836 0.487 0.589 0.317 0.852 ...
#> .. $ diff_filt:'data.frame': 635 obs. of 16 variables:
#> .. ..$ GeneName : chr [1:635] "Rrs1" "Mcm3" "Ogfrl1" "Smap1" ...
#> .. ..$ B-A_FC : num [1:635] -2.06 -1.45 1.07 2.96 -1.24 2.41 -1.63 2.41 53.5 1.63 ...
#> .. ..$ B-A_logFC : num [1:635] -1.04 -0.539 0.102 1.57 -0.312 1.27 -0.706 1.27 5.74 0.708 ...
#> .. ..$ B-A_tstat : num [1:635] -2.86 -1.87 0.292 2.01 -1.1 0.706 -1.9 3.2 3.64 1.91 ...
#> .. ..$ B-A_pval : num [1:635] 0.0147 0.0869 0.775 0.0684 0.295 0.494 0.0827 0.00784 0.00354 0.0804 ...
#> .. ..$ B-A_adjpval: num [1:635] 0.276 0.544 0.96 0.499 0.789 0.875 0.535 0.21 0.142 0.53 ...
#> .. ..$ C-A_FC : num [1:635] -2.71 -2.45 -3.78 5.68 2.69 36.4 3.13 2.74 287 -6.39 ...
#> .. ..$ C-A_logFC : num [1:635] -1.44 -1.29 -1.92 2.51 1.43 5.19 1.64 1.46 8.17 -2.68 ...
#> .. ..$ C-A_tstat : num [1:635] -3.83 -4.33 -4.04 3.59 5.99 3.13 5.63 3.97 5.42 -3.83 ...
#> .. ..$ C-A_pval : num [1:635] 2.48e-03 1.03e-03 1.71e-03 3.82e-03 6.94e-05 8.86e-03 1.22e-04 1.94e-03 1.68e-04 2.50e-03 ...
#> .. ..$ C-A_adjpval: num [1:635] 0.0368 0.0224 0.0303 0.047 0.00463 0.0764 0.00644 0.0322 0.00784 0.0369 ...
#> .. ..$ B-C_FC : num [1:635] 1.32 1.69 4.05 -1.92 -3.34 -15.1 -5.1 -1.14 -5.37 10.4 ...
#> .. ..$ B-C_logFC : num [1:635] 0.399 0.755 2.02 -0.938 -1.74 -3.92 -2.35 -0.189 -2.42 3.38 ...
#> .. ..$ B-C_tstat : num [1:635] 0.941 2.38 4.03 -1.75 -6.98 -4.23 -7.09 -0.583 -4.01 4.57 ...
#> .. ..$ B-C_pval : num [1:635] 3.66e-01 3.53e-02 1.76e-03 1.07e-01 1.66e-05 1.23e-03 1.44e-05 5.71e-01 1.82e-03 6.84e-04 ...
#> .. ..$ B-C_adjpval: num [1:635] 0.666 0.23 0.055 0.378 0.0033 0.0455 0.00309 0.816 0.0562 0.0333 ...